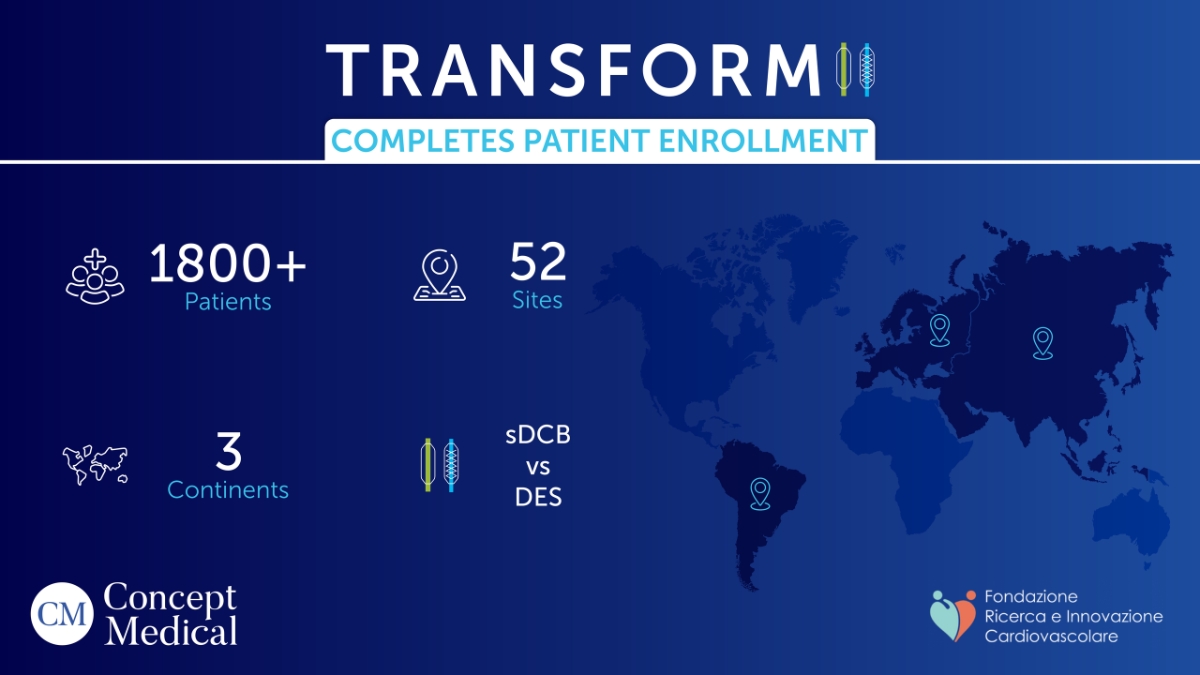
New Delhi [India], June 14: The Fondazione Ricerca e Innovazione Cardiovascolare (RIC) has successfully finished patient enrolment for TRANSFORM II, one of the most ambitious and extensive randomised controlled trials (RCTs) ever carried out in the field of cardiovascular care, at a pivotal point for the future of interventional cardiology. In order to treat de-novo coronary artery disease, this international project is contrasting the popular everolimus-eluting stent (EES) with the innovative MagicTouch Sirolimus-Coated Balloon (SCB).
The trial, helmed by the distinguished Prof. Bernardo Cortese of the Harrington Heart & Vascular Institute (Cleveland, USA), reached its enrollment target of 1,832 patients on June 6, 2025. The culmination of more than three years of relentless dedication, TRANSFORM II spans 52 global centres across Europe, Asia, and South America, making it one of the most geographically expansive RCTs focused on drug-coated balloon technology.
A New Chapter for Coronary Intervention
The sirolimus-coated balloon has emerged as a compelling alternative to drug-eluting stents for small vessel coronary artery disease. Traditionally, stents, while life-saving, effectively cage arteries, a strategy not without long-term complications in small-diameter vessels. TRANSFORM II is rewriting this playbook.
“After 3.5 years, we’ve reached a huge milestone. This trial wasn’t just large; it was ambitious, spanning continents and medical cultures,” said Prof. Cortese. “We aim to drive the adoption of drug-coated balloons, especially sirolimus-based options, using real-world patient data and comparing them with the most established DES technology available.”
High-Stakes Science in Action
With 1,832 patients now onboard, TRANSFORM II is positioned to deliver high-impact data. The trial compares the MagicTouch SCB, a product of Concept Medical, against the gold-standard everolimus-eluting stent.
Key trial parameters include:
- Primary Endpoint: Target Lesion Failure (TLF) at 12 months
- Patient Population: Those with de-novo coronary lesions in vessels 2.0 mm–3.5 mm in diameter
- Monitoring Period: Up to 60 months
- Sub-study: Optical Coherence Tomography (OCT) in 70 patients to assess arterial healing and patency at 9 months
These results are poised to fill a critical evidence gap in the treatment of small vessel coronary artery disease.
The Global Cardiovascular Community Watches Closely
Dr. Manish Doshi, Founder & Managing Director of Concept Medical, called the enrollment milestone “a testament to international collaboration and clinical rigour.”
“We’re proud to support what may become a landmark study in cardiology,” said Dr. Doshi. “Sirolimus-coated balloon technology is not just an alternative; it could be a paradigm shift. This trial’s scale and scientific design speak volumes about our collective vision to elevate patient care.”
Concept Medical’s MagicTouch SCB is already CE Mark-approved and carries Breakthrough Device Designation from the U.S. FDA for treating both small coronary vessels and in-stent restenosis.
Why This Matters: Beyond the Numbers
Roughly 80% of patients undergoing percutaneous coronary interventions (PCI) fall into the vessel size category being studied in TRANSFORM II. In these smaller arteries, placing a metallic stent can sometimes do more harm than good in the long term. That’s where sirolimus-coated balloon technology comes into its own: it delivers the anti-restenotic drug without leaving a permanent implant.
The MagicTouch SCB uses Nanoluté technology, delivering sub-micron sirolimus particles into the vessel wall via a biocompatible carrier. This ensures deep penetration and drug efficacy without structural compromise.
technology, delivering sub-micron sirolimus particles into the vessel wall via a biocompatible carrier. This ensures deep penetration and drug efficacy without structural compromise.
As Prof. Cortese aptly put it: “We are paving the route for the modern angioplasty era.”
A Look Ahead
With enrollment complete, attention now turns to patient monitoring and eventual results. The 12-month outcomes will be closely watched by regulatory bodies, interventional cardiologists, and healthcare policymakers. A favourable comparison for SCBs could catalyse a major shift in global treatment protocols, offering millions of patients a safer, non-stent-based alternative.
And the momentum is building. As new ARC guidelines support broader use of drug-coated balloons, TRANSFORM II may serve as the definitive evidence base to accelerate that adoption.
Conclusion: A Trial That Could Transform
TRANSFORM II stands as more than a clinical trial; it is a vision for the future of cardiovascular care. With the sirolimus-coated balloon by Fondazione Ricerca e Innovazione Cardiovascolare now standing toe-to-toe with traditional stent therapy, the upcoming data could well revolutionise the practice of angioplasty and reframe how the medical community approaches coronary disease. As the world waits for results, one thing is clear: this trial isn’t just about numbers, it’s about redefining care, one artery at a time.
The article is for general information purposes only and should not be construed as professional medical advice. Always consult your doctor before taking any step.